
As a result, a decision tree has been created by the Committee for Veterinary Medicinal Products (CVMP) with the goal of allowing regulators to conduct a full risk assessment by January 2023, demonstrating compliance with European Pharmacopeia General Monograph 2619 as seen below. This process has brought the focus on digitalization as the best solution to face the new production volumes and quality. As a matter of fact, manufacturers are trying to find solutions to automatize their production and data handling systems, in order to reduce human impact and its possible negative consequences.
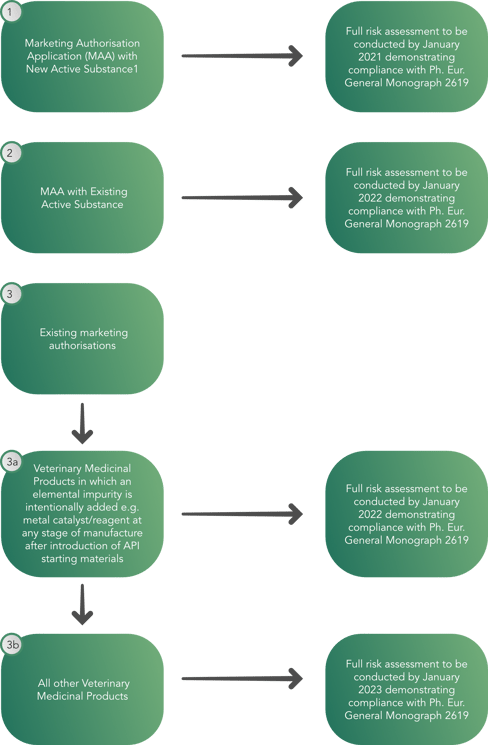
The decision tree provides a phased implementation approach to the veterinary medicinal products containing:
- Chemical and biological/biotechnological substances
- Synthetic and semi-synthetic antibiotics
- Synthetic peptides of low molecular weight
The decision tree does not apply to:
- Veterinary herbal products, radiopharmaceuticals, immunological products, veterinary medicinal products designed for gene therapy, regenerative medicine, tissue engineering, blood product therapy and phage therapy or to elements that are intentionally included in a veterinary medicinal product for therapeutic benefit.
So the question is, when is a risk assessment required?
A risk assessment will be required when the identification of potential elemental impurities are found. These impurities could be derived from intentionally added catalysts and inorganic reagents, or that are unintentionally present in active substances and/or excipients, as well as coming from the manufacturing equipment or the packaging of the medicinal product.
There are currently three approaches to construct the risk assessment:
- The medicinal product approach
- The component approach
- The combination of the medicinal product and component approaches
The control strategy of the risk assessment should be based on quality risk management guidelines, but for veterinary medicinal products, the principles for risk identification, risk analysis and risk evaluation can be used for:
- Identification of known and potential sources of elemental impurities that may find their way into the veterinary medicinal product.
- Evaluation of the presence of a particular elemental impurity in the veterinary medicinal product by determining the observed or predicted level of the impurity and comparing it with the acceptance limits.
- Summary and documentation of the risk assessment, which should be available at the manufacturing site for review and inspection.
With the European Pharmacopoeia General Monograph 2619 being updated in January 2023, further guidance of expected regulatory actions will be more fully explained and published. PQE Group can provide support to your business and ensure the full compliance with the new guidelines in veterinary medicinal products. Our services portfolio guarantees a comprehensive solution to cover all of your needs by using our Glocal approach -- we work at a local level while maintaining a global mindset. We offer:
- Webinars and trainings to educate your workforce on the new concepts of the recent release
- Support for integrating new concepts and methodologies in your QMS
- Workshops, Assessments & Gap Analysis oriented to prepare for the update of the CVMP
- Writing of new procedures and/or updating existing ones
- Support for the application of new concepts to real (pilot) projects, systems, equipment and infrastructures
FONT: European Medicines Agency
REF: EMA/CVMP/QWP/631010/2017-REV2 - Committee for Medicinal Products for Veterinary Use (CVMP)