Pharmacovigilance Systems in Pharma Companies
Pharmacovigilance is defined as the "science and activities relating to the detection, assessment, understanding, and prevention of adverse events or any other medicine-related problem." Pharmaceutical companies, as Marketing Authorization Holders, have specific obligations in this regard, defined by their respective legal framework. In particular, they must have a pharmacovigilance system suitable for collecting information on medicinal products and designed to monitor the safety of authorized medicinal products and detect any changes to their risk-benefit balance. In addition to a regulatory requirement, a good pharmacovigilance system represents a fundamental strategy to ensure patient safety, improve the company's reputation, and guide the development of increasingly safe and effective therapies.
Post- Marketing Pharmacovigilance
The safety profile of a medicinal product available on the market must be constantly monitored because the population it comes into contact with is much more complex than the limited sample of patients undergoing pre-marketing clinical trials. New variables come into play, such as pre-existing diseases, drug interactions in multi-drug therapy, genetic and dietary factors, special groups (pregnant women, children, elderly) and the duration of treatments (e.g., chronic use), which can bring forth new safety and efficacy information (e.g., rare but serious adverse reactions, potential risks, etc.).
Economic Considerations
Pharmacoeconomic studies have demonstrated that the financial cost to a nation of adverse drug reactions (hospitalization, surgery, lost productivity, etc.) is higher than the cost of a pharmacovigilance system. In light of this, a pharmacovigilance system becomes essential for the cost-effective use of medicines in all countries.
Spontaneous Reports
In the history of pharmacovigilance, healthcare professionals (HCP) have been the major providers of case reports of suspected ADRs. However, underreporting of ADRs remains a major problem. To overcome this deficiency and have a reasonable amount of safety data, pharmaceutical companies should contribute in spreading the culture of reporting among healthcare professionals. This should emphasize the value of pharmacovigilance as an integral part of clinical practices and promote growing awareness of the industry’s fundamental role in improving the understanding of diseases and the safer use of medicines.
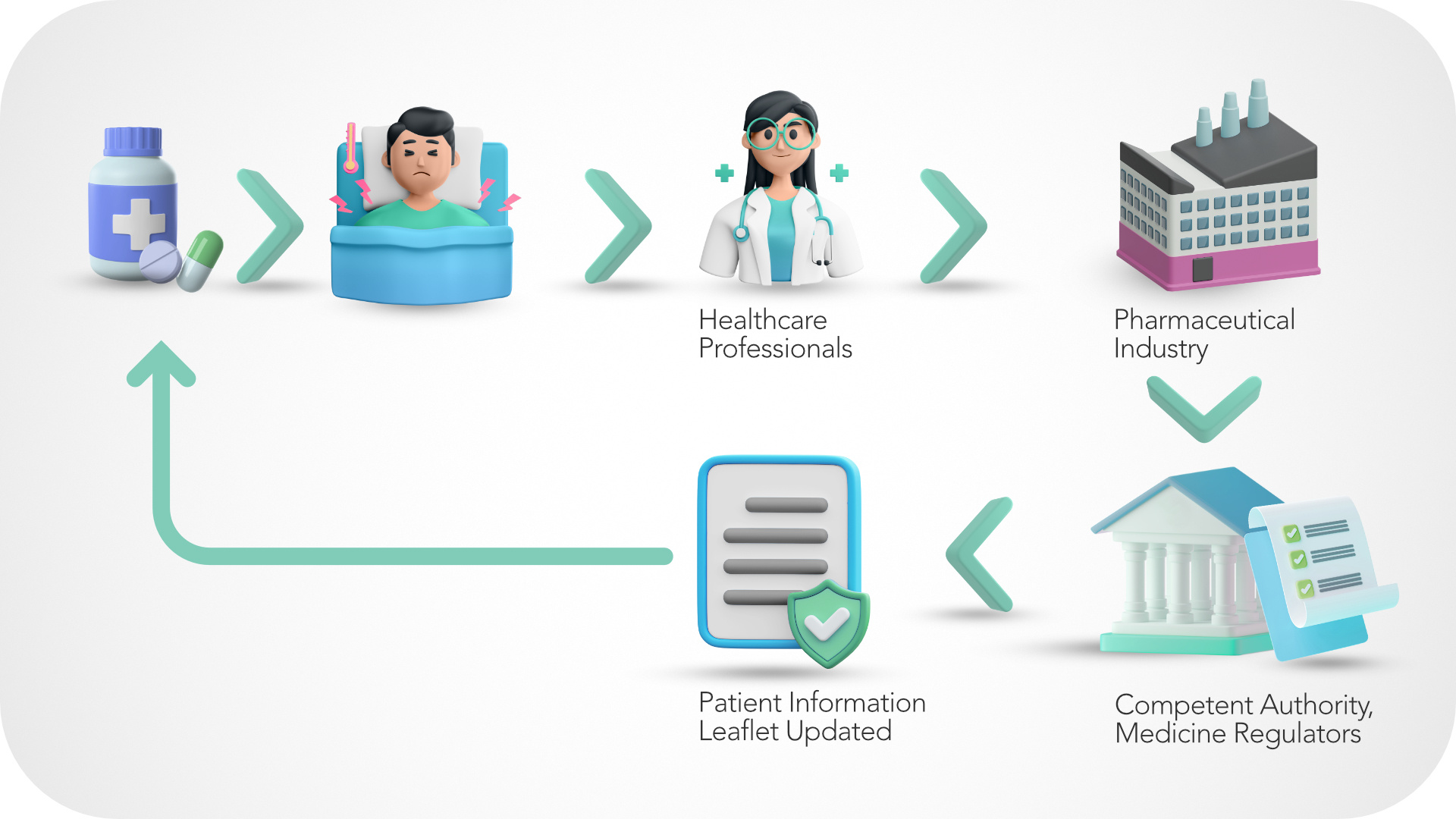
The Central Role of Healthcare Professionals in Pharmacovigilance
In the collective imagination, the healthcare professional is the reference figure we turn to when we have a health problem. Healthcare professionals represent much more in clinical practice as they are the connecting figure between pharmaceutical companies, national pharmacovigilance centers, and patients. They play a key role in preserving public health, which is among the objectives of pharmacovigilance. Furthermore, they possess the right knowledge, skills, and readiness to identify, recognize, manage, and prevent adverse drug reactions.
Sources:
- World Health Organization (WHO). The importance of pharmacovigilance: safety monitoring of medicinal products. Genève: WHO; 2002
- World Health Organization (WHO). The safety of medicines in public health programmes: pharmacovigilance an essential tool. Genève: WHO; 2006