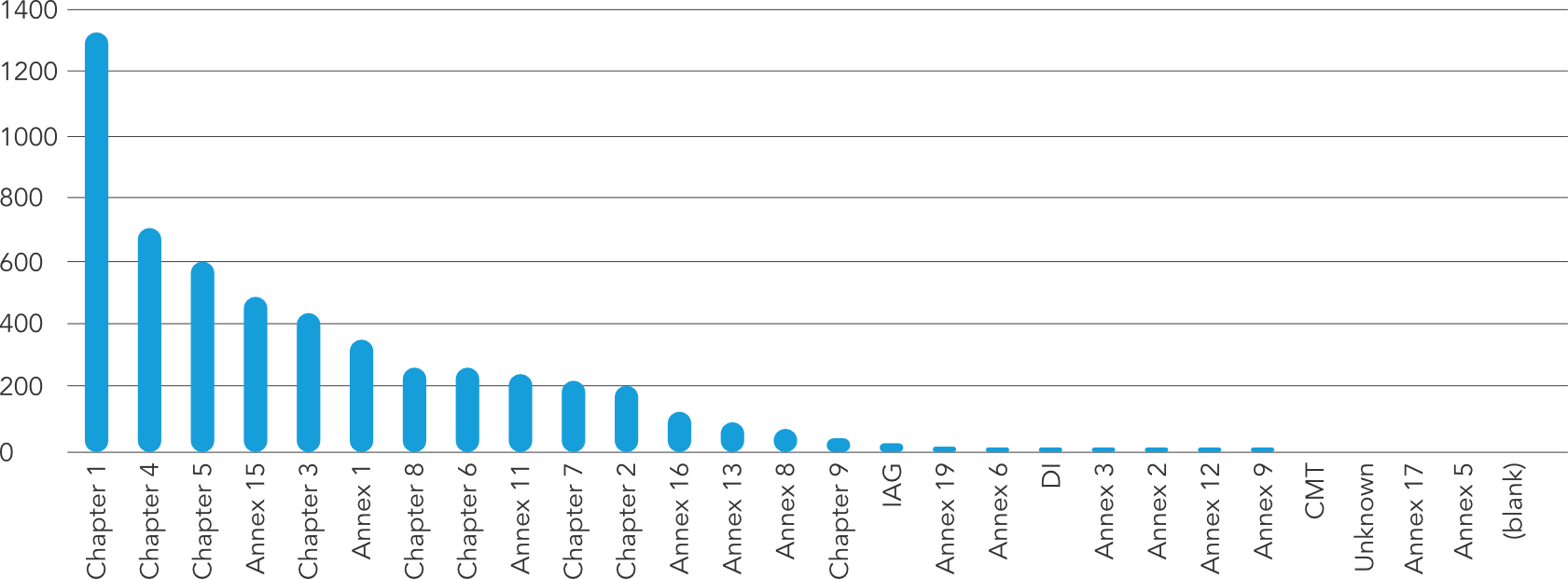
According to the graph sourced from MHRA's annual GMP inspection deficiencies report for 2019, the top 10 most cited deficiencies were:
1. Chapter 1 - Pharmaceutical Quality System
The most-cited finding in 2019 appeared in Chapter 1, which covers the requirements for a pharmaceutical quality system. The main issues identified were related to the implementation and effectiveness of the quality system, including the identification and management of risks. To address these findings, companies should ensure that they have a robust quality system in place, with appropriate documentation and procedures to manage risks and ensure compliance with GMP requirements.
2. Chapter 4 - Documentation
Chapter 4 outlines the requirements for documentation, and contained the second most-cited finding in 2019. The main issues identified were related to the completeness and accuracy of documentation, including batch records, validation protocols, and standard operating procedures. Companies can improve their compliance with this chapter by ensuring that their documentation is complete, accurate, and up-to-date, with appropriate controls in place for document revision and change management.
3. Chapter 5 - Production
Chapter 5 outlines the requirements for production activities, and contained the third most-cited finding in 2019. The main issues identified were related to the validation of production processes and the control of critical process parameters. Companies can address these findings by ensuring that they have appropriate validation protocols in place for their production processes, and that critical process parameters are monitored and controlled in accordance with GMP requirements.
5. Annex 15 - Qualification and Validation
Annex 15 outlines the requirements for qualification and validation, and was the fourth most-cited finding in 2019. The main issues identified were related to the completeness and effectiveness of the company's validation activities, including the validation of computerized systems. Companies can address these findings by ensuring that they have appropriate validation protocols in place for their processes and systems, and that they regularly review and update these protocols as needed to ensure ongoing compliance.
6. Chapter 3 - Premises and Equipment
The sixth most-cited finding in 2019 was related to Chapter 3, which covers the requirements for premises and equipment. The main issues identified were related to the maintenance and qualification of equipment, as well as the cleanliness and condition of facilities. To address these findings, companies should ensure that their equipment is properly maintained and qualified, with appropriate documentation and validation records. In addition, facilities should be regularly cleaned and maintained to ensure that they meet GMP requirements.
7. Annex 1 - Manufacture of Sterile Medicinal Products
The top findings in 2019 related to Annex 1 outline the requirements for the manufacture of sterile medicinal products. The main issues identified were related to the design and operation of clean rooms and associated facilities. Companies can improve their compliance with this Annex by ensuring that they have effective control measures in place for their clean rooms, including appropriate environmental monitoring, cleaning procedures, and staff training.
9. Chapter 8 - Complaints and Product Recall
Chapter 8 covers the requirements for handling complaints and product recalls, and had the seventh most-cited finding in 2019. The main issues identified were related to the timeliness and effectiveness of the company's recall procedures, as well as the investigation and management of complaints. Companies can address these findings by ensuring that they have robust procedures in place for handling complaints and product recalls, including effective communication with regulatory bodies and customers.
10. Chapter 6 - Quality Control
Chapter 6 covers the requirements for quality control, and had the eighth most-cited finding in 2019. The main issues identified were related to the qualification and training of personnel involved in quality control activities, as well as the effectiveness of the quality control system. Companies can address these findings by ensuring that their quality control personnel are properly qualified and trained, and that they have appropriate procedures in place to manage their quality control activities.
11. Annex 11 – Computerized Systems
Annex 11 was the ninth most-cited finding in the MHRA's 2019 report. The main issues identified were related to the validation and control of computerized systems, including data integrity and security. Companies can improve their compliance with this Annex by ensuring that they have appropriate procedures in place for the validation, control, and maintenance of computerized systems. This includes implementing controls to ensure data integrity, such as access controls, audit trails, and data backup and recovery procedures. Companies should also ensure that their computerized systems are validated in accordance with GMP requirements and that appropriate change control procedures are in place to manage any changes to these systems.
12. Chapter 7 - Outsourced Activities
Chapter 7 outlines the requirements for outsourced activities, which was the ninth most-cited finding in 2019. The main issues identified were related to the oversight and management of outsourced activities, including the selection and qualification of suppliers. Companies can improve their compliance with this Chapter by ensuring that they have appropriate processes in place for selecting and qualifying suppliers, and that they have effective oversight mechanisms in place to monitor the performance of their outsourced activities.
Final thoughts
Staying up-to-date on the latest regulatory requirements and inspection findings is critical for companies in the pharma industry to maintain compliance and ensure the quality of their products. By focusing on the top findings from the MHRA's 2019 report, companies can identify areas for improvement and implement measures to address these findings. This can help companies to avoid costly compliance issues and maintain their reputation as a trusted provider of high-quality pharmaceutical products.
At PQE, we provide expert consulting services to support pharmaceutical companies in achieving and maintaining compliance with regulatory requirements. Our team of highly experienced professionals can help companies assess their current quality management systems, identify gaps, and develop effective strategies for improvement. We also provide training and guidance to help companies stay up-to-date with the latest regulatory developments and industry trends.
With our support, companies can address the top ten MHRA inspection findings and ensure compliance with GMP regulations, while maintaining their focus on innovation and growth. Contact us today to learn more about how we can help your company achieve regulatory compliance and improve its overall quality management system.
Download the e-book