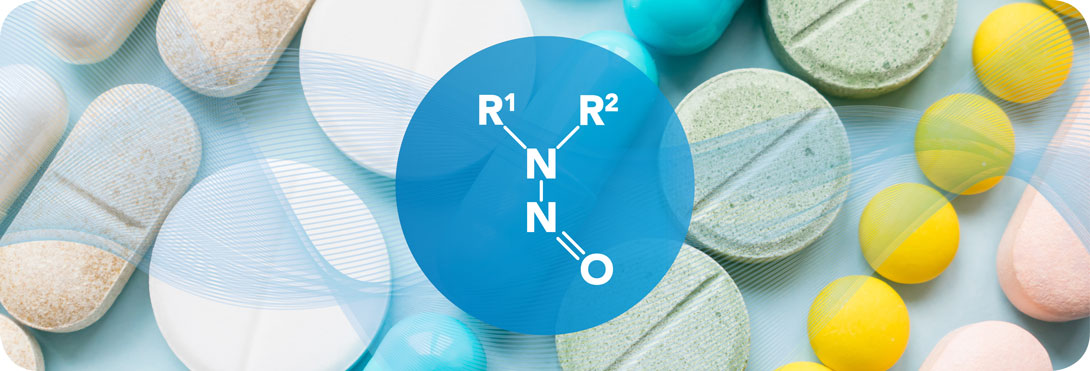
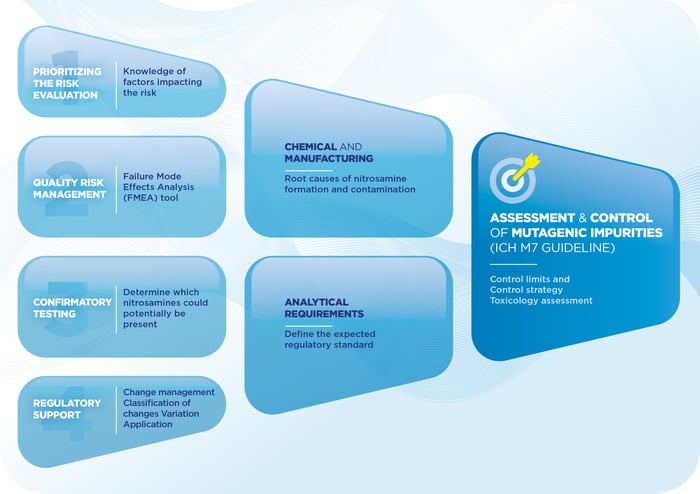
PQE Supports Marketing Authorization Holders, Manufacturers of API and Manufacturers of Finished products.Our experts have significant expertise with the quality risk management principles (ICH Q9 guideline) and principles of assessment and control of mutagenic impurities in pharmaceuticals (ICH M7 guideline): we provide full support on Risk Evaluation, Risk Management and Regulatory Requirements (ICH Q9 & ICH M7 guidelines).
Check out our Regulatory Affairs and Quality Compliance Services, and contact our team to request a meeting with our experts.
Connect with us